Achieving Mass Production of CECSi Cells
| Penetrating Keratoplasty | Endothelial Keratoplasty (DMEK, DSAEK etc.) |
Allogenic CLS001 Cell Transplantation |
|
|---|---|---|---|
 |
 |
 |
|
| Procedure | Full suture of graft | Adhesion of graft using air pressure | Replacement cells are adhered by gravity |
| Complications | Immune rejection 8-40% Glaucoma 27% Infection 2-12% |
Immune rejection < 40% Glaucoma 0-54% Infection, donor contamination≒1% |
Immune rejection < 10% (assumed) |
| Other | Astigmatism≒100% | Adhesion failure 1-26% | Must maintain a face-down position for three hours after transplantation |
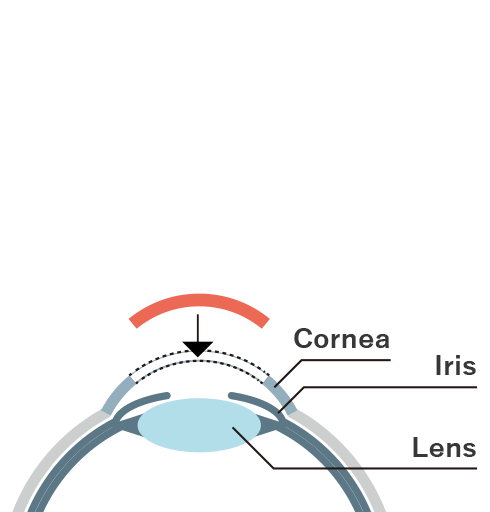
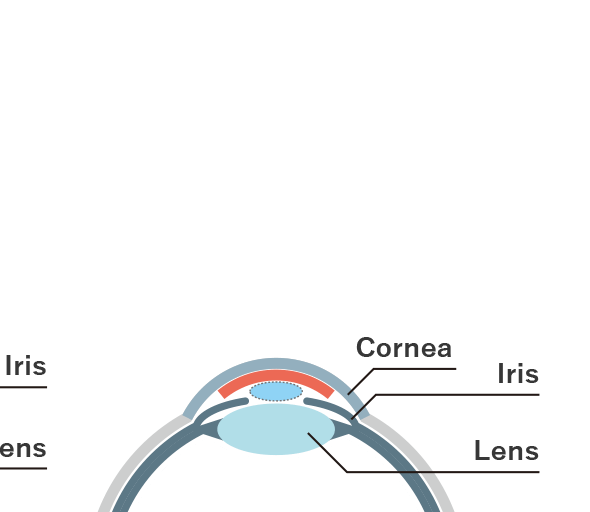
Bullous keratopathy can be hereditary (Fuchs’ corneal endothelial degeneration) or due to trauma caused during intraocular surgery such as cataract surgery. It decreases the corneal endothelial cells and causes an excessive influx of fluid to the cornea, resulting in edema (swelling) and clouding. Until recently, corneal transplants were typically performed for bullous keratopathy, including Penetrating Keratoplasty, transplantation of the entire cornea, and Endothelial Keratoplasty, transplantation of the corneal endothelium (Figure 1, left and center). Because the cornea does not contain blood vessels and is less prone to rejection than other organ transplants, allogeneic corneal transplants (transplantation of another person’s tissue) are performed without blood type or other matching.
On the other hand, conventional corneal transplantation methods still present various problems such as postoperative infection due to large wounds, astigmatism, increased intraocular pressure, and poor adhesion of the graft. In addition, it is estimated that there are approximately 10,000 patients on the waiting list for corneal transplants in Japan, but only less than 2,000 donor eyes are available in Japan each year, so patients have had to wait more than a year for their turn to receive a corneal transplant.
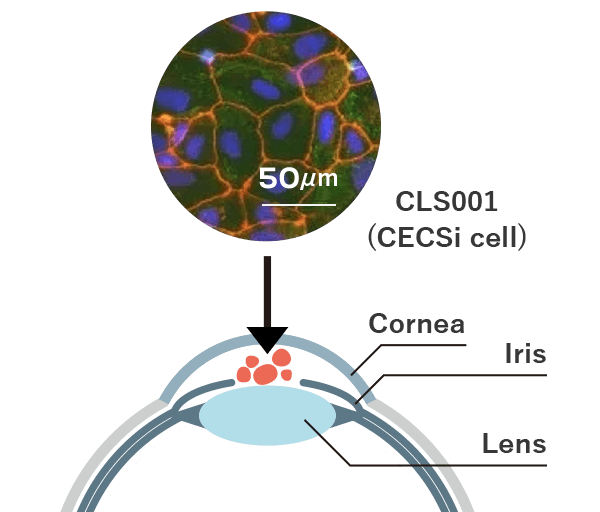
Research by Professor Shimmura and Professor Hatou of Keio University has demonstrated that producing CECSi cells, corneal endothelial cell substitute from iPSC – which function in the same was as corneal endothelial cells – and injecting them with a syringe into the posterior surface of the cornea has considerable potential as an effective treatment for bullous keratopathy (Figure 1, right).
This method has the potential to significantly reduce complications because the wound is much smaller than in existing corneal transplant procedures. In addition, it is now possible to cryopreserve these cells at the final stage of production, which means that the corneal endothelial replacement cells needed for transplantation therapy can be produced and stored in large quantities in advance and promptly transplanted as necessary.
As part of the first-in-human investigator-initiated clinical research, we plan to receive clinical grade iPS cells from the Regenerative Medicine iPS Cell Stock Project established by CiRAF, and to use these cells to create transplantable graft cells.
(Keio University School of Medicine, Keio University Hospital Press Release )
Injection of CECSi Cells
into the Eye Reduces Patient Burden
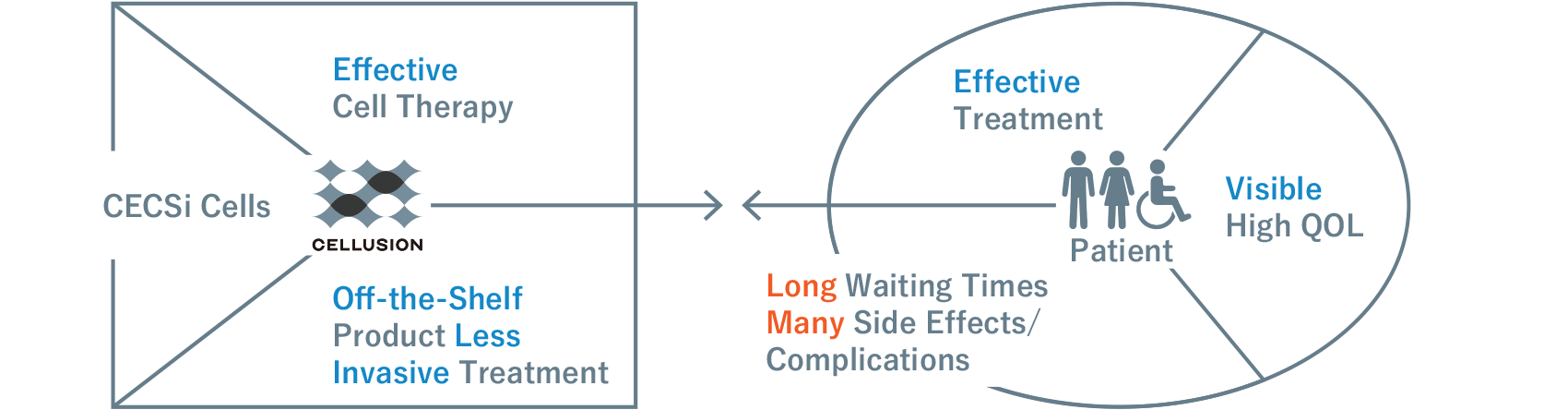
Using iPS cells generated from skin, blood, and other sources from healthy individuals, Cellusion succeeded in producing CECSi cells, corneal endothelial cell substitute from iPSC, that function in the same way as corneal endothelial cells. CECSi cells were found to have considerable potential as an effective treatment for bullous keratopathy when a suspension of the cells is injected into the eye with a syringe and adheres to the cornea. This restores the normal thickness and transparency of the cornea.
The figure on the right shows a comparison between existing therapies and the new method of cell transplantation with CLS001. Although CLS001 requires the patient to remain in a face-down position for about three hours after the suspension has been injected to allow the replacement cells to adhere, the wound is much smaller than in existing corneal transplantation methods, resulting in fewer complications, less invasiveness, and less stress for the patient. It is also a simpler procedure than conventional corneal transplantation and eliminates the risk of contamination, which is inevitable in donor-based medicine.
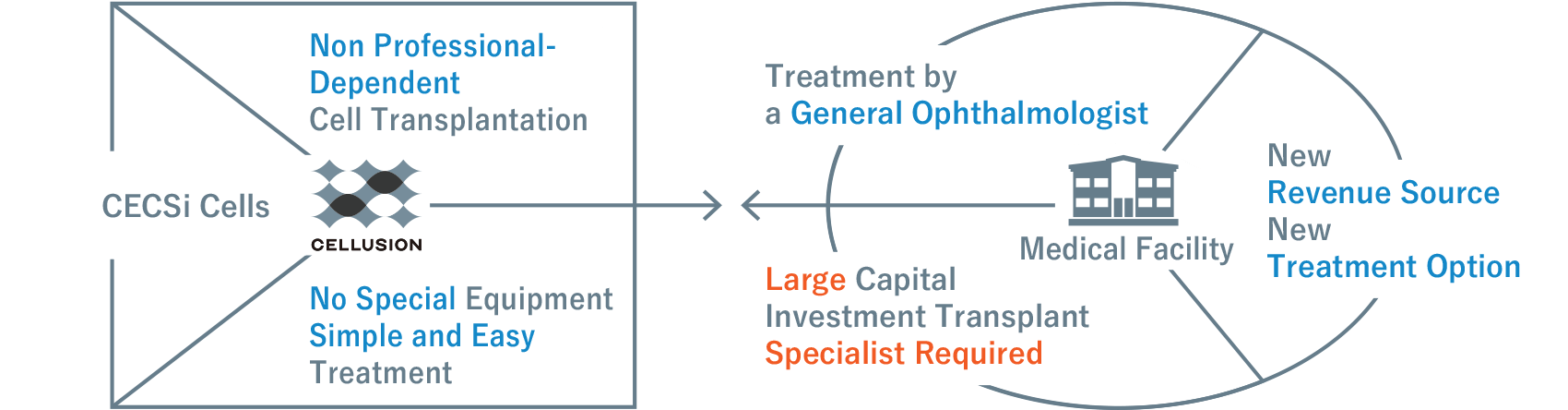
CLS001 also offers other advantages. Cellusion possesses a proprietary manufacturing technology that directly differentiates iPS cells into corneal endothelial replacement cells without intermediates. This ensures stable cell quality and enables cells to be mass produced quickly. In addition, as the generated cells can be frozen and stored for a certain period, they can be transported to various locations and used when needed by the patient.
Given the simplicity of injecting the suspension, it will be possible for ophthalmology clinics nationwide to provide the treatment once it becomes commercially available, even if a corneal transplant specialist or specialized facility cannot be secured.